Dr. Daniel Weinstein
Professor and Dean of Faculty, School of Mathematics and Natural Sciences
Ph.D., The Rockefeller University
Office: NSB E -124 Tel: 718-997-4552
Laboratory: NSB E-121; 718-997-4258
E-mail: daniel.weinstein@qc.cuny.edu
Research interests:
My laboratory’s effort focuses on elucidating the signaling mechanisms underlying germ layer formation and patterning in the vertebrate embryo.

Research interests:
Regulation of pluripotency in the early vertebrate embryo
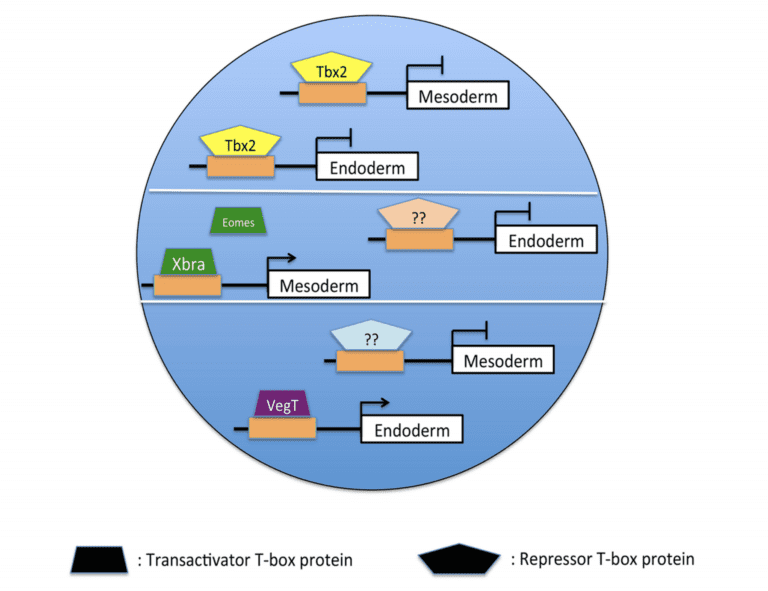
Developmental biology is fundamentally concerned with the processes by which the single cell of the fertilized egg gives rise during embryogenesis to the organized distribution of the hundreds of cell types in the adult organism. The specification and separation of the primary germ layers (ectoderm, mesoderm, and endoderm) are early and essential steps in the development of the vertebrate embryo. A well-supported model has emerged that emphasizes the requirement for inductive interactions in the formation of both mesoderm and endoderm. It has been widely assumed that specification of the vertebrate ectoderm occurs essentially by default in the absence of both mesoderm and endoderm induction; our group and others have found, however, that the differentiation of the ectoderm requires the active suppression of mesodermal and endodermal fate. Recent studies from our lab demonstrate that transcriptional repression by the T-box DNA-binding protein Tbx2 limits the response of presumptive ectodermal cells to mesoderm- and endoderm-promoting cues in embryos of the frog Xenopus laevis. Current projects aim to establish the gene regulatory and signaling mechanisms through which Tbx2 suppresses inappropriate germ layer formation, and thus restricts embryonic cell pluripotency, in vivo.
A model of Tbx2-mediated ectodermal specification. Tbx2 binds to and represses target genes in animal pole progenitor cells, thereby inhibiting mesoderm and endoderm formation. In the marginal zone and vegetal pole, other T-box proteins bind to and activate an overlapping set of target genes, stimulating mesodermal and endodermal differentiation.
Patterning of the anterior ectoderm
The cement gland, as its name implies, secretes a sticky mucus that allows amphibian larvae to adhere to hard surfaces before young tadpoles are able to swim freely in the aquatic environment. The cement gland is an ectodermal structure located at the anterior end of the Xenopus laevis tadpole, at the border between dorsal (neural) and ventral (epidermal) fates; it has long been used as a model to study inter- and intracellular regulation during early embryonic patterning and differentiation. It has been shown that an intermediate level of Bone Morphogenetic Protein (BMP) signaling is essential for cement gland formation; several transcription factors have also been linked to the differentiation of this organ. Recent findings in our lab suggest that the homeodomain-containing transcription factor Pitx1 functions both to limit local BMP signaling within the cement gland primordium and to recruit the BMP signal transducer Smad1 to activate direct downstream targets; current work seeks to test and refine this model. In related studies, we are examining the role of Apolipoprotein CI, a lipid-binding protein implicated in atherosclerosis and Alzheimer’s disease in humans, in the regulation of other anterior fates including the forebrain, sensory placodes, and cranial neural crest.
Selected Publications:
Research Articles
Reich, S., Kayastha, P., Teegala, S., and Weinstein, D.C. (2020). Tbx2 mediates dorsal patterning and germ layer suppression through inhibition of BMP/GDF and Activin/Nodal signaling. BMC Molecular and Cell Biology 21:39. https://doi.org/10.1186/s12860-020-00282-1.
Teegala, S., Chauhan, R., Lei, E., and Weinstein, D.C. (2018). Tbx2 is required for the suppression of mesendoderm during early Xenopus development. Dev Dyn 247, 903-913.
Jin, Y. and Weinstein, D.C. (2018). Pitx1 regulates cement gland development in Xenopus laevis through activation of transcriptional targets and inhibition of BMP signaling. Dev. Biol., 437, 41-49.
Sridharan, J., Haremaki, T., and Weinstein, D.C. (2018). Cloning and spatiotemporal expression of Xenopus laevis Apolipoprotein CI . PLoS ONE 13(1): e0191470. https://doi.org/10.1371/journal.pone.0191470
Grumolato, L., Liu, G., Haremaki, T., Mungamuri, S.K., Mong, P., Akiri, G., Lopez-Bergami, P., Arita, A., Anouar, Y., Mlodzik, M., Ronai, Z.A., Brody, J., Weinstein, D.C., and Aaronson, S.A. (2013).β-cateinin-independent activation of TCF1/LEF1 in human hematopoietic tumor cells through interaction with ATF2 transcription factors. PLoS Genet, 9, 1-13.
Haremaki, T., and Weinstein, D.C. (2012). Eif4a3 is required for accurate splicing of the Xenopus laevis ryanodine receptorpre-mRNA. Dev. Biol. 372, 103-110.
Kim, K., Lake, B.B., Haremaki, T, Weinstein, D.C., and Sokol, S.Y. (2012). Rab11 regulates planar polarity and migratory behavior of multiciliated cells in Xenopus embryonic epidermis. Dev. Dyn. 241, 1385-1395.
Sridharan, J., Haremaki, T., Jin, Y., Teegala, S., and Weinstein, D.C. (2012). Xmab21l3 mediates dorsoventral patterning in Xenopus laevis. Mech. Dev.129, 136-146.
Haremaki, T., Sridharan, J., Dvora, S., and Weinstein, D.C. (2010). Regulation of vertebrate embryogenesis by the Exon Junction Complex core component Eif4a3. Dev. Dyn. 239, 1977-1987.
Haremaki, T., and Weinstein, D.C. (2009). Xmc mediates Xctr1-independent morphogenesis in Xenopus laevis. Dev. Dyn. 238, 2382-2387.
Haremaki, T., Fraser, S.T., Kuo, Y-M., Baron, M.H., and Weinstein, D.C. (2007). Vertebrate Ctr1 coordinates morphogenesis and progenitor cell fate and regulates embryonic stem cell differentiation. Proc. Natl. Acad. Sci. USA. 104, 12029-12034.
Suri, C., Haremaki, T., and Weinstein, D.C. (2005). Xema, a foxi-class gene expressed in the gastrula stage Xenopus ectoderm, is required for the suppression of mesendoderm formation. Development 132, 2733-2742.
Suri, C., Haremaki, T., and Weinstein, D.C. (2004). Inhibition of mesodermal fate by Xenopus HNF3β/FoxA2. Dev. Biol. 265, 90-104.
Hama, J., Suri, C., Haremaki, T., and Weinstein, D.C. (2002). The molecular basis of Src kinase specificity during vertebrate mesoderm formation. J. Biol. Chem. 277, 19806-19810.
Hama, J., Xu, H., Goldfarb, M., and Weinstein, D.C. (2001). SNT-1/FRS2α physically interacts with Laloo and mediates mesoderm induction by Fibroblast Growth Factor. Mech. Dev. 10, 195-204.
Song, Y., Cohler, A.N., and Weinstein, D.C. (2001). Regulation of Laloo by the Xenopus C-terminal Src kinase (Xcsk) during early vertebrate development. Oncogene 20, 5210-5214.
Weinstein, D.C., Marden, J., Carnevali, F., and Hemmati-Brivanlou, A. (1998). FGF-mediated mesoderm induction involves the Src-family kinase Laloo. Nature 394, 904-908.
Weinstein, D.C., Honore, E., and Hemmati-Brivanlou, A. (1997). Epidermal induction and inhibition of neural fate by translation initiation factor 4AIII. Development 124, 4235-4242.
Weinstein, D.C., Ruiz i Altaba, A., Chen, W.S., Hoodless, P., Prezioso, V.R., Jessell, T.M., and Darnell, J.E., Jr. (1994). The winged helix transcription factor HNF-3β is required for notochord development in the mouse embryo. Cell 78, 575-588.
Book Chapters and Reviews
Reich, S., and Weinstein, D.C. (2019). Repression of inappropriate gene expression in the vertebrate embryonic ectoderm. Genes 10(11), 895; https://doi.org/10.3390/genes10110895
Wee, N.K.Y., Weinstein, D.C., Fraser, S.T., and Assinder, S.J. (2013). The mammalian copper transporters CTR1 and CTR2 and their roles in development and disease. The International Journal of Biochemistry and Cell Biology 45, 960-963.
Weinstein, D.C. (2005). Mesodermal differentiation: signal integration during development. Science STKE 2005, tr 23.
Weinstein, D.C. (2004). Function of the winged helix transcription factor HNF3β/FoxA2 during gastrulation. In Stern, C. (ed.), Gastrulation, Cold Spring Harbor Laboratory Press, New York, 563-570.
Weinstein, D.C., and Hemmati-Brivanlou, A. (1999). Neural Induction. Annu. Rev. Cell. Dev. Biol. 15, 411-433.